gravimetric determination of water of crystallisation by volatilization method|volatilization gravimeter chart : maker Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight. Resultado da 累計発行部数2000万部を突破し、第45回講談社漫画賞少年部門も受賞した、講談社「週刊少年マガジン」連載中の原作・金城宗幸、漫画・ノ村優介による大人気サッカーコミックス「ブルーロック」。2022年10月8日(土)深夜1:30~テレビ朝日系全国ネット“NUMAnimation ”にて放送開始
{plog:ftitle_list}
A Calebito é uma marca estabelecida e reconhecida na região, oferecendo uma experiência única para seus clientes.
The best way to appreciate the theoretical and practical details discussed in this section is to carefully examine a typical volatilization gravimetric method. Although each method is unique, the determination of . We can also use the results from volatilization gravimetry analysis to determine the percentage of water of crystallization. The percentage of water of crystallization tells us the .Silver, gold, and platinum can be determined by burning the organic sample, leaving a metallic residue of Ag, Au, or Pt. Other metals are determined by adding HNO 3 before combustion, leaving a residue of the metal oxide. Volatilization .Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight.
In precipitation methods of gravimetric analysis, a substance is dissolved in water and converted into an insoluble product by the addition of a suitable reagent.We can determine the mass of water of crystallization in a salt, such as this, using volatilization gravimetry. If we know the mass of the hydrated salt and we remove the water of crystallization using thermal energy by heating, the water will escape as a vapor leaving behind a white powder. In this lesson, we will learn how to use volatilization gravimetry to calculate the quantity of analyte in a sample or determine the formula of a hydrated compound.
Classifying Quantitative Analytical Methods • Gravimetric Methods: Determine the mass of the analyte or some compound chemically related to it. Analyte: the compound or species to be . A second approach to gravimetry is to thermally or chemically decompose the sample and measure the resulting change in its mass. Alternatively, we can trap and weigh a .Gravimetry includes all analytical methods in which the analytical signal is a measurement of mass or a change in mass. When you step on a scale after exercising you are making, in a .
volatilization gravimetry chemistry
Thermogravimetry. One method for determining the products of a thermal decomposition is to monitor the sample’s mass as a function of temperature, a process called thermogravimetry.Figure 8.3.1 shows a typical thermogram in which each change in mass—each “step” in the thermogram—represents the loss of a volatile product. As the following example .12 Gravimetric Methods of Analysis Gravimetric analysis: based upon mass measurements highly accurate and precise. --- Isolation (pure known substance) & weighing *Advantages 1. accuracy 2. single fundamental chemical precipitation reaction ex: Cl- + Ag+ → AgCl(s) 3. require no accuracy known standard solution 12A Precipitation Gravimetry 1. Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, 2007) provides an approved method for determining the moisture content of flour. A preweighed sample is heated for one hour in a 130 .
Gravimetric analysis is an analytical technique used for the quantitative determination of an analyte based on the mass of a solid. . The analytical technique of gravimetric volatilization separates the masses using thermal or chemical energy to determine their masses. . water, and sulfur dioxide. This method has been successfully used to .The precipitation method is the one used for the determination of the amount of calcium in water. Using this method, an excess of oxalic acid, H 2 C 2 O 4, is added to a measured, known volume of water.By adding a reagent, here ammonium oxalate, the calcium will precipitate as calcium oxalate.The proper reagent, when added to aqueous solution, will produce highly .
brix refractometer grapes
Because the release of a volatile species is an essential part of these methods, we classify them collectively as volatilization gravimetric methods of analysis. 8.4: Particulate Gravimetry Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction.define the volatilization method in gravimetric analysis, describe the experimental procedure involved in volatilization gravimetry, connect the loss of mass of a hydrated compound with the loss of water molecules from the compound, calculate the percentage of water of crystallization in a hydrated compound,The simple gravimetric analysis involves simple heating, precipitation, drying and separation of sample in order to find out volatile and non-volatile components. The analyte may be separated from the sample by converting it into gas and the mass of gas serves as measure of analyte concentration this process is known as volatilization .Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participate in a chemical reaction. . Particulate gravimetry is important in the environmental analysis of water, air, and soil samples. The analysis for suspended solids in water samples, .
4. Types of Gravimetric Analyses: • There are two main types of gravimetric analyses: A) Precipitation – analyte must first be converted to a solid (precipitate) by precipitation with an appropriate reagent. The precipitates from solution is filtered, washed, purified (if necessary) and weighed. B) Volatilization – In this method the analyte or its decomposition .It is a crucial method within gravimetric analysis, complementing other techniques like volatilization gravimetry. Solubility Product : The solubility product (Ksp) is an equilibrium constant that represents the extent to which a sparingly soluble .Gravimetric Methods Chapter Overview 8A Overview of Gravimetric Methods 8B Precipitation Gravimetry 8C Volatilization Gravimetry 8D Particulate Gravimetry 8E Key Terms 8F Chapter Summary . The change in the absorbent’s mass provides a direct determination of the amount of water in the sample. An easier approach is to weigh the sample of
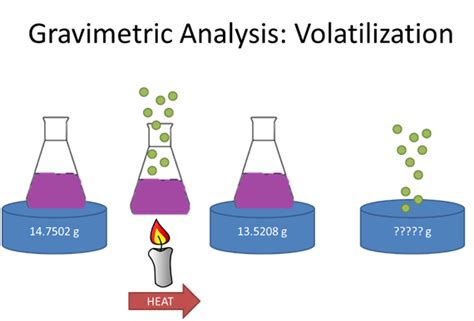
Indirect method volatilization gravimetry. In indirect methods we can define weight of the rest of substance after full removal of a defined volatile component. It is used for moisture determination or to find water of crystallization, etc. %( ) 1 2 100%; a m m Z A m 1 –mass of the sample before drying m 2 –mass of the sample after drying
What is Gravimetric Analysis? Gravimetric analysis is a method in analytical chemistry to determine the quantity of an analyte based on the mass of a solid. Example: Measuring the solids suspended in the water sample – Once a known volume of water is filtered, the collected solids are weighed. The principle of Gravimetric Analysis: Water of crystallisation is when some compounds can form crystals which have water as part of their structure; A compound that contains water of crystallisation is called a hydrated compound; The water of crystallisation is separated from the main formula by a dot when writing the chemical formula of hydrated compounds . E.g. hydrated copper(II) sulfate is .Ask the Chatbot a Question Ask the Chatbot a Question gravimetric analysis, a method of quantitative chemical analysis in which the constituent sought is converted into a substance (of known composition) that can be separated from the sample and weighed. The steps commonly followed in gravimetric analysis are (1) preparation of a solution containing a known weight of .Volatilization method • In this method the analyte or its decomposition . • Example, Water can be separated from most inorganic . compounds by ignition. . Gravimetric analysis avoids problems with temperature fluctuations, calibration errors, and other problems associated with .
• Indirect analyses based on the weight of the residue remaining after volatilization are commonly used in determining moisture Cont’ • Organic analysis –the combustion products are passed through preweighed tubes containing appropriate absorbents – provides a direct indication • Also used to determine biomass in water and . Elemental weight can be calculated based on the formula of the compound and the atomic weight of the element the elements or compounds contained in it are carried out in various ways, such as .
Gravimetric Methods of Analysis . 1/35 Gravimetric methods: The . quantitative methods. that are based on determining the . mass. . Volatilization gravimetry: The analyte is separated from other constituents of a sample by converting it to a gas .Gravimetric analysis is based on the measurement of the mass of a substance of known composition that is chemically related to the analyte. Gravimetric analysis includes precipitation, volatilization and electrodeposition methods. In precipitation gravimetry of the analyte is carried out by the use of inorganic or organic
(b) a gravimetric precipitation method and a gravimetric volatilization method. *(c) precipitation and coprecipitation. (d) peptization and coagulation of a colloid. *(e) occlusion and mixed-crystal formation. (f) nucleation and particle growth.
Determination of Water of crystallization in copper sulphate hydrous CuSO4.XH2O using Volatilization method Theory: Water of crystallization is the water forming part of crystal structure of certain materials, known as crystalline hydrate. The contents of .
Add 50 mL distilled water to the sample in each beaker, then 5 mL of 6 M HCl and then add another 200 mL distilled water. Cover the beakers with the 100 mm watch glasses and store them in your cabinet until it is convenient to proceed with the determination.A {eq}4.256 g {/eq} sample of the commercial product was heated strongly to drive off the waters of crystallization. The anhydrous residue weighed {eq}2.256 g {/eq}.As a result, the change in weight after treating with HF and H2SO4 is due only to the loss of SiF4. 8C.2 Quantitative Applications Unlike precipitation gravimetry, which is rarely used as a standard method of analysis, volatilization gravimetric methods continue to play an important role in chemical analysis.
volatilization gravimetric method
brix refractometer homebrew
brix refractometer honey
A brief description of the manhua TALES OF DEMONS AND .
gravimetric determination of water of crystallisation by volatilization method|volatilization gravimeter chart